CBD has established itself as one of the fastest growing food supplement products in the UK with some estimates valuing the sector at around £300m. Millions of Brits are consuming it, and the industry has already outgrown sales of vitamin C and D combined. CBD sales are projected to hit £1bn by 2025, which is equivalent to the entire UK herbal supplement market in 2016.
It’s no wonder then that retailers are keen to stock products containing this popular ingredient. However, retailers may be surprised to learn that companies selling CBD products as food are operating in a grey area and many could even be illegal due to the amount of controlled substances they contain.
The UK is the only market in the world where a clear regulatory framework exists for commercial adoption of CBD giving retailers the opportunity to participate in this huge market. However, they must ensure that their procurement process is able to identify the products that are fully safe and legal.
The UK’s Food Standards Agency (FSA) has given CBD companies until 31 March 2021 to have a validated novel foods application for each of their products or else risk being removed from shelves. This is a complex and costly process so it’s critical that retailers ensure brands they stock have the necessary infrastructure, expertise and resources to meet this deadline.
A recent report by a government advisory group, the Committee on Toxicity, concluded that there are some remaining safety concerns regarding human consumption of CBD; the ACI’s members are engaged in allaying these concerns through investing in safety studies to prove that CBD is safe to consume as a food supplement.
A blind testing exercise reported in The Times last year identified over one third of the products tested contained illegal amounts of the controlled substance THC, one third contained less CBD than advertised and one product, sold through a major high street retail chain, even contained 0% CBD. Another’s residual alcohol content was so high it should have been classified as an alcoholic beverage. Results like these demonstrate the level of due diligence retailers need to adhere to when assessing which CBD products to stock.
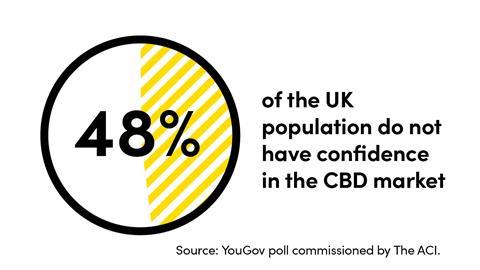
To help retailers and consumers to identify products which meet the necessary regulatory, safety and legal requirements The Association for the Cannabinoid Industry (The ACI) runs a Safety Certification programme.

In addition, experts from the ACI have identified three key factors retailers must take into account when evaluating CBD products.
● Regulatory framework - make sure CBD companies are not misleading you about their CBD products.
● Controlled substances - ensure you do not fall foul of the Misuse of Drugs Act.
● Food safety - avoid food scare scandals.
Regulatory framework
At a European level, CBD is classified as what is known as a “novel food”. A novel food is an ingredient that has not been consumed en masse before May 1997 (the date the novel foods catalog was created). Some argue that because CBD is found in hemp oil it has been consumed for many years before this date, but the majority of CBD products on the market contain CBD that has been extracted using techniques that were not used for CBD extraction prior to 1997, hence the novel foods classification.
A novel food can only be sold if the company has a novel foods authorisation. As yet, no company has a novel foods authorisation for CBD anywhere in Europe.
You may be wondering why is it possible to sell CBD products in the UK? In the UK, the FSA have always agreed that CBD is a novel food. However, they took a hands-off approach due to the market being so big and so many consumers were already relying upon CBD for its perceived therapeutic benefit. Driving CBD into the black market would have been a disaster.
On 13 February 2020 the FSA issued a statement granting CBD companies until 31 March 2021 to get their CBD products validated or else they would not be legal to sell. Validation is the first stage of the novel foods authorisation process.
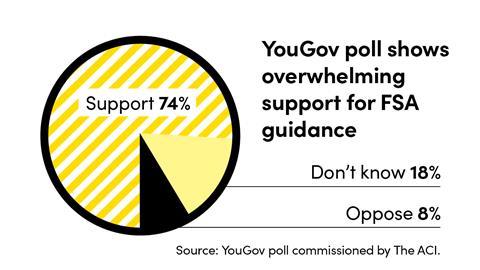
This window of opportunity only applies to products that were already for sale on the market prior to 13 February 2020. Any new product would have to get a full novel foods authorisation, a process that could take an estimated three years.
When retailers are reviewing the CBD products they stock it is critical that they are aware of what the novel foods status is of these products otherwise they could be in a position where they can no longer sell them legally after FSA’s deadline. The ACI have engaged with Trading Standards and they are aware of this situation and willing to enforce once this deadline expires.
Controlled substances
CBD is derived from hemp flowers. Hemp is a variety of cannabis that has very low amounts of THC. In the UK, THC is illegal to cultivate, process or sell without a license from the Home Office. Only CBD containing non detectable amounts of THC are legal to sell. Retailers selling CBD products that contain THC could run foul of law enforcement officers because of this controlled substance they contain.
There is a persistent rumour that 0.3% THC is acceptable, however this is not true. This misinformation stems from the fact that hemp grown in the UK can contain up to 0.3% THC. However, that rule does not extend to finished products.
Food safety
If thoughts of the police or Trading Standards coming knocking at retailers’ doors are not worrying enough, then nothing grabs headlines more than food safety scandals. To understand the risks of CBD products it’s important to understand the supply chain of how this ingredient is produced.
As mentioned above, CBD is derived from hemp plants. A resilient crop, hemp can grow in very poor, or even contaminated, soil. It can even be grown in places to extract contamination from the land, and is remarkably currently being grown around Chernobyl to mop up after the nuclear power plant meltdown. Think of the cannabis plant stems as a straw and imagine the flowers as a sponge. As it grows, the plant sucks up the impurities through the straw and they end up in the dense flowering buds. Once harvested, CBD is extracted from the flowers, along with any other impurities that could be there.
Recently CBD products in the US were recalled after testing by the Florida Department of Agriculture and Consumer Services found high levels of lead in a batch of tinctures. Had these products been digested they could cause various symptoms such as pain, nausea and kidney damage. Many consumers find CBD helpful for ailments, and rely on retailers to vet the quality of the product. It is of utmost importance that retailers are confident that CBD is safe for consumption.
The ACI can help
So what is a retailer to do? CBD is a popular product and millions of consumers are taking it for the benefits they gain. Retailers must ensure that their procurement process is able to weed out any products that could potentially land them in legal troubles or be a hazard to their customers.
The ACI exists to nurture a safe, legal and well regulated CBD market in the UK. We have helped guide major global retailers to ensure that their procurement process is able to allow them to act as responsible gatekeepers to this industry. In doing so, we will help the UK lead the world in this exciting market whilst helping millions of consumers as they seek the therapeutic relief they gain from CBD.
The ACI exists to ensure all stakeholders in the industry are well educated on the issues relating to CBD. To be sure your procurement process enables you to stock only safe and legal CBD products please contact The ACI.
The Association for the Cannabinoid Industry (The ACI) is committed to nurturing a safe, legal and well regulated CBD market in the UK. Visit https://www.theaci.co.uk/ for more information.






















No comments yet